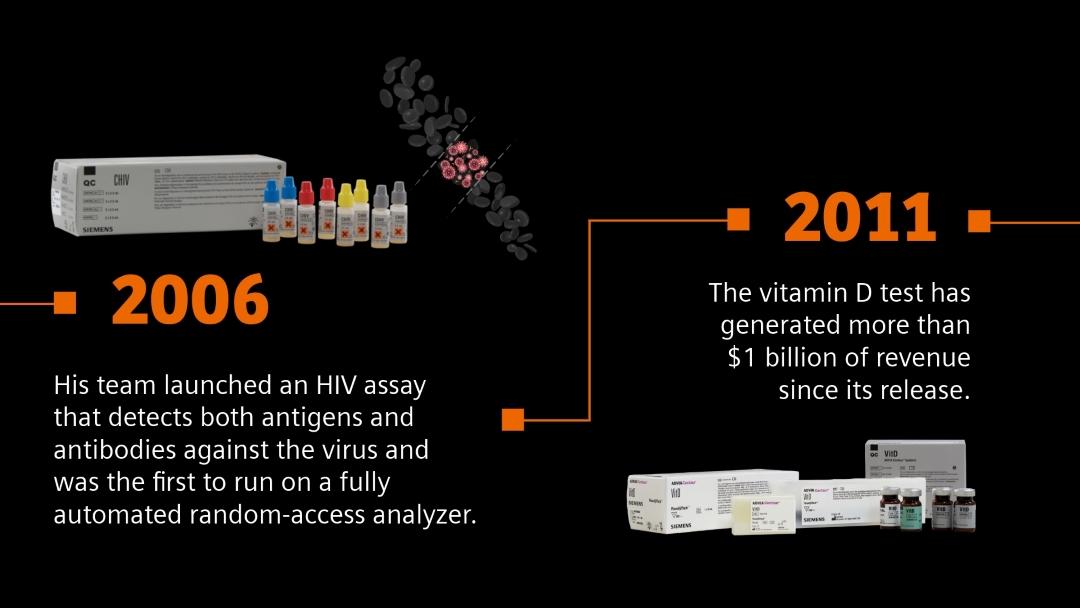
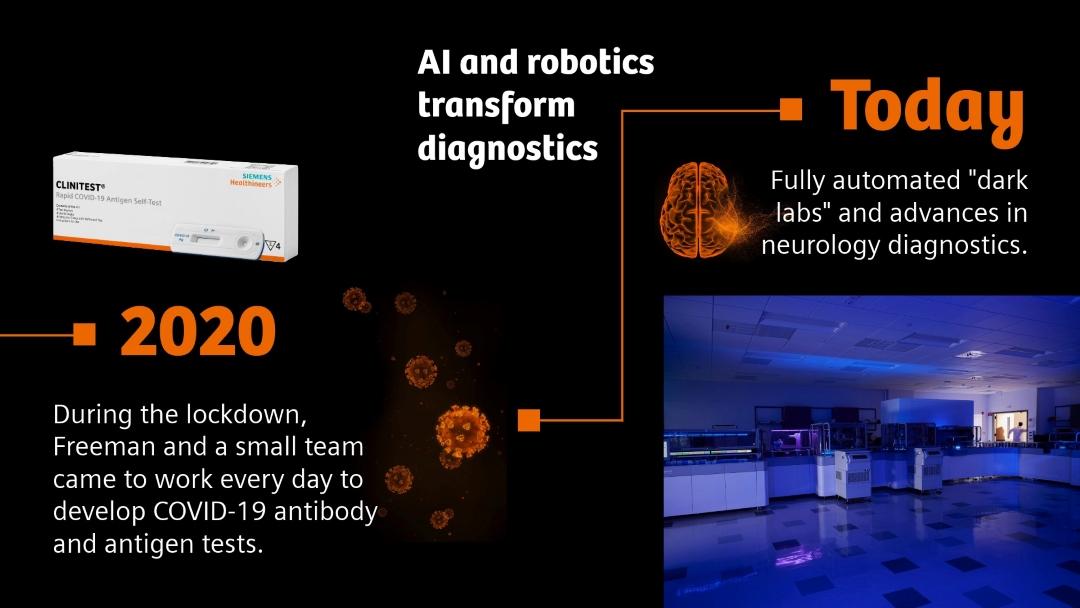
From HIV to Alzheimer’s:
A life dedicated to developing blood tests
Developing an assay is an iterative process that requires several optimization steps: we make an assay formulation, test it, and if, for example, the precision does not meet the requirement, we iterate/optimize to the next formulation. There’s a lot of art to it.
Jim Freeman, Head of Core Lab Solutions R&D at Siemens Healthineers








Ideas come through dialogue and collaboration. You need people who bring a fresh perspective and think outside the box to innovate on instrument design and assay development.
Jim Freeman, Head of Core Lab Solutions R&D at Siemens Healthineers

Become part of the team



The product names and/or brands referred to are the property of their respective trademark holders. The products/features mentioned herein are under development and/or are not commercially available in all countries. Their future availability cannot be guaranteed.
References
[1] World Health Organization. Dementia fact sheet [Internet]. Who.int. March 31, 2025. Available from: https://www.who.int/en/news-room/fact-sheets/detail/dementia. Last accessed October 1, 2025.
[2] Siemens Healthcare Diagnostics Inc. Decoding doctors’ decisions. How system friction and patient agency affect physicians — and what this means for lab testing [Internet]. July 2025. Available from: https://www.siemens-healthineers.com/en-us/press-room/press-releases/adlm-2025-physician-survey
[3] Siemens Healthcare Diagnostics Inc. Clinical labs in critical condition: What lab professionals reveal about the impact of workforce shortage on patient care [Internet]. July 2024. Available from: https://www.siemens-healthineers.com/en-us/lab-shortage-impact-survey-2024