History
A 49-year-old male presented with extensive liver metastases. 68Ga-DOTATATE PET/CT imaging was used to confirm that the mass was the result of a neuroendocrine tumor (NET). The patient was subsequently referred for radionuclide therapy with 177Lu DOTATATE. High tracer uptake within the liver metastases demonstrated on the PET/CT suggested a high concentration of somatostatin receptors. As such, the patient underwent 2 cycles of radionuclide therapy, each with 7.4 GBq of 177Lu DOTATATE. A follow-up 68Ga-DOTATATE PET/CT showed a significant amount of central necrosis within the large liver lesions and a slight reduction in size in the smaller metastatic lesions. In view of the partial response to therapy, along with substantial volume of residual-functioning, traceravid tumor in the liver and absence of bone or nodal or soft tissue metastases, the patient was treated with a third therapeutic dose of 7.4 GBq 177Lu DOTATATE.
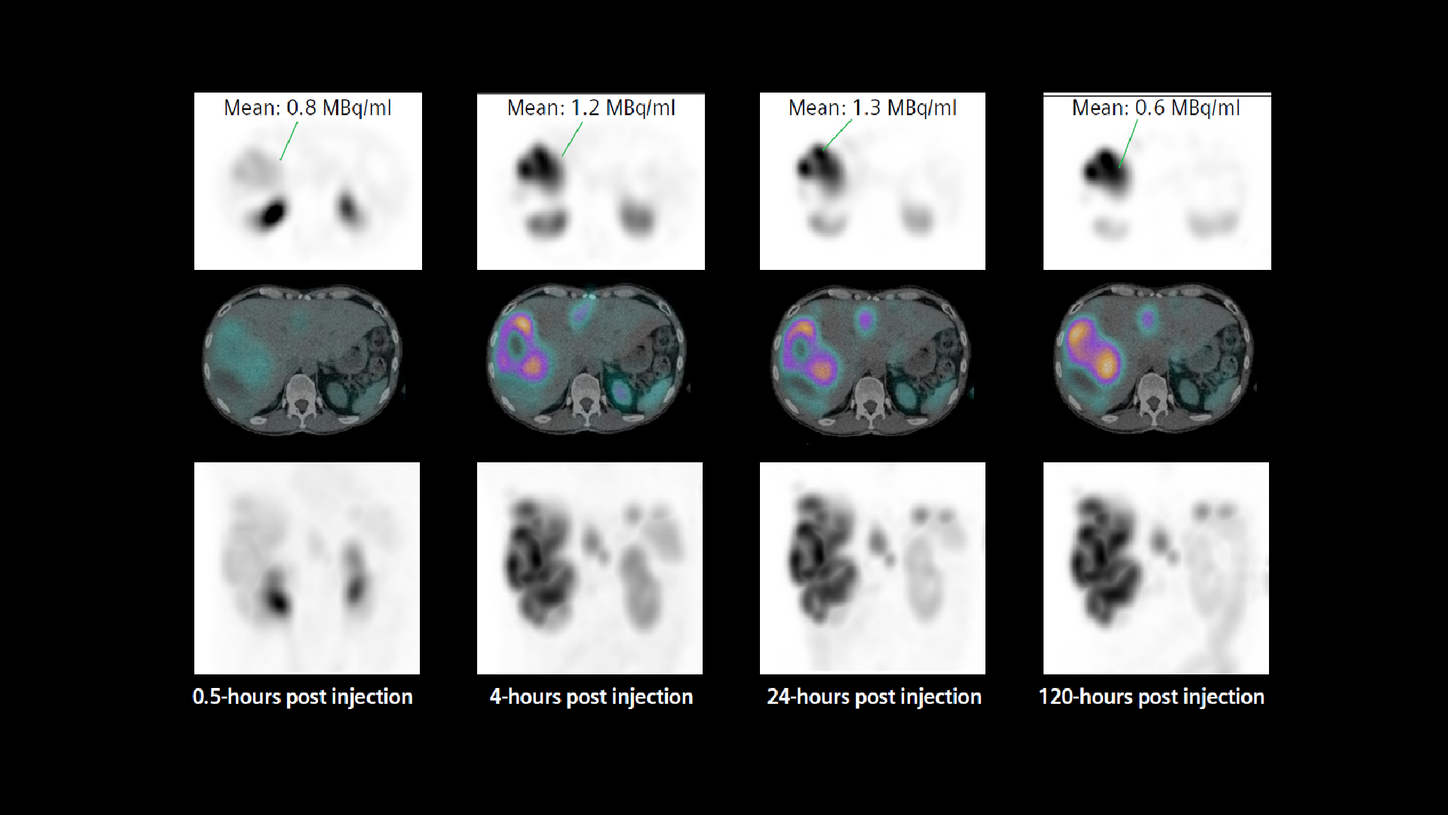
The therapy dose was delivered as an intravenous infusion along with the standard regime of amino acid administration prior to and during therapy. Thirty minutes after commencing administration of the first therapeutic dose of 177Lu DOTATATE, the patient underwent a SPECT/CT study, which was followed by subsequent SPECT/CT studies performed at 4-, 24-, and 120-hours post injection in order to assess tracer concentration in tumor and critical organs, such as the kidneys, across four time points. The exams were performed on a Symbia Intevo™ SPECT/CT scanner using xSPECT Quant™, which involved using a NIST-traceable 75Se point source of known activity for calibration in order to accurately quantify the studies.
xSPECT Quant acquisition was performed using 60 stops with 20 seconds per stop. The study was reconstructed using OSCGM reconstruction incorporating attenuation and scatter correction. The volume of interest (VOI) was generated using fused SPECT/CT images over the tumor and critical organs, such as the kidneys, were evaluated to assess volumes and absolute tracer concentration within the VOIs.
Findings
Visual inspection of sequential, fourtime point SPECT/CT (Figures 1 and 2) demonstrated high tracer uptake in multiple liver metastases with progressive increase in uptake until 24 hours and very slow clearance as evident in substantial remaining tracer uptake by 120 hours. This suggests high initial uptake and slow washout in the tumor, which indicates a possibility of high tumor dose delivery due to increased tumor tracer retention time. However, the kidneys showed normal cortical uptake. Fast clearance suggests lower renal tracer retention time with the possibility of low renal dose.
xSPECT Quant enabled the evaluation of changes in tracer concentration in the kidneys and tumor. xSPECT Quant and CT data fed into research dosimetry software enable automated generation of VOIs for the tumor as well as critical organs such as the kidneys, liver, and spleen, in order to estimate absolute tracer concentration in each VOI in kBq/ml. Tumor, kidney, and liver volumes can be derived from VOIs generated from fused SPECT/CT images with high accuracy. Corresponding tracer concentrations attained from xSPECT Quant lead to the estimation of absolute tracer concentration in kBq within the VOI. Such estimations from SPECT/CT data across four time points may be used to determine absorbed dose to the tumor and critical organs like the kidneys, liver, and spleen.
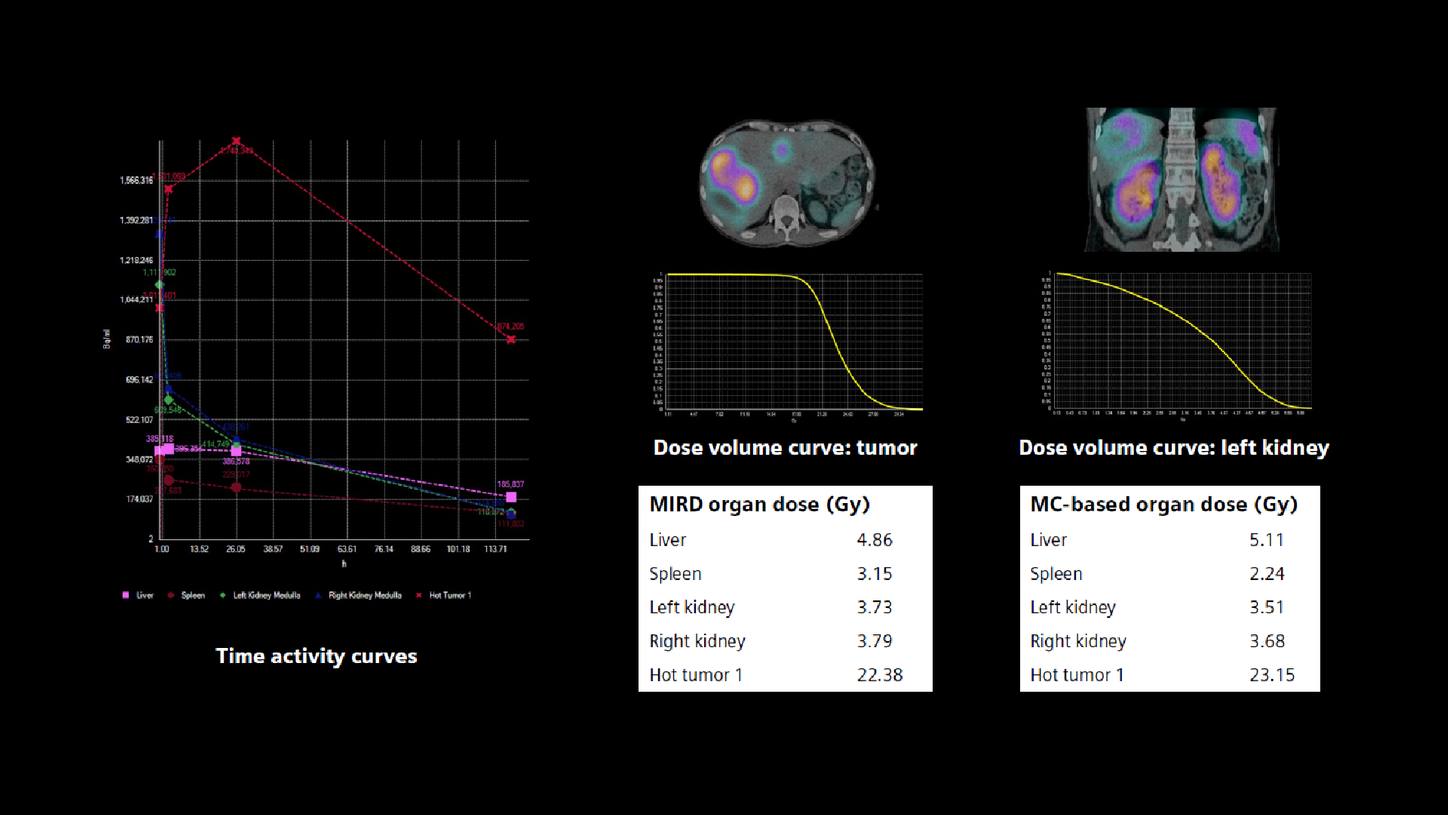
Furthermore, multiple time-point images subsequently converted to percentage-of-administered radioactivity concentration enable time activity curves to be generated, mono or bi-exponential curve fitting to be performed, as well as subsequent Medical Internal Radiation Dose (MIRD) or voxel-based dosimetry.
The multi-time point xSPECT Quant data was processed by research dosimetry software to yield dose estimates. The four time-point studies were accurately registered to each other and VOIs were generated and aligned across multiple time points to accurately assess quantitative changes in absolute tracer concentration across time and yield time-activity curves.
Comments
Most well-differentiated NETs express somatostatin receptors, particularly SST2 receptors, which result in high uptake of radiolabeled somatostatin analogs, such as 177Lu DOTATATE, that are used for treatment of patients with advanced metastatic NETs with peptide receptor radionuclide therapy (PRRT). Apart from β emission, 177Lu DOTATATE is also a gamma emitter, which enables planar and SPECT imaging to be performed following therapeutic dose. This allows for the demonstration of tumor uptake and estimation of tumor and critical organ dose along with a prediction of therapeutic efficacy, in addition to the need and feasibility of multiple therapies. The standard therapy approach with 177Lu DOTATATE is for four cycles to be administered at 8–12 week intervals with each therapy dose
being 7–7.4 GBq. The absorbed dose to the tumor is restricted by the maximum acceptable absorbed dose to the kidneys and bone marrow, hence limiting the total number of administered therapy cycles.
A reliable dosimetry method is mandatory in order to ensure optimal administration of therapy dose without exceeding toxicity limits. Therapeutic dosimetry might help better predict the maximal absorbed dose to the tumors while minimizing radiation burden to the critical organs. In order to achieve a reliable dosimetry estimate, accurate and precise activity quantification is essential.
Although the majority of the experience of dosimetry for 177Lu-DOTATATE therapy is based on sequential whole-body planar scintigraphic acquisition, the overlap between liver and renal activity on planar images may lead to errors in defining renal and liver volumes leading to inaccurate quantification of tracer concentration for dosimetry. Often a SPECT or SPECT/CT acquisition for 3D delineation and segmentation of liver and kidneys is performed as part of the dosimetry acquisition in order to accurately define the relative activity of the liver and kidney volumes, thereby improving the accuracy of the time activity curves. Sequential SPECT/CT acquisitions, instead of planar and along with the use of 3D-voxel-based dosimetry, has been shown to improve the dosimetric accuracy and in some cases have shown significant difference in tumor and critical organ dose between 2D and 3D dosimetry. A study comparing planar and SPECT/CT-based 3D dosimetry for 111In-, 90Y-, and 177Lu-labelled somatostatin analogs in metastatic NET showed consistently higher dose with 3D dosimetry compared to planar 2D dosimetry.1 The mean dose was 0.45 mGy/MBq with 3D dosimetry compared to 0.31 mGy/MBq for planar.
Conclusion
The present study demonstrates the value of 3D-voxel-based dosimetry derived from Monte Carlo calculations using sequential SPECT/CT studies. It also uses standard MIRD dosimetry with automated organ and tumor segmentations obtained from the SPECT/CT studies. Organ segmentations for liver, spleen, and kidneys were based on CT data, while tumor segmentations were based on SPECT data with VOI generated using a threshold of 50% of maximum uptake. Time activity curves generated from automatically segmented organs or tumor were fitted using biexponential curve fit, from which total tracer residence time was generated from the area under the curve for dosimetric evaluation.
xSPECT Quant enables accurate estimation of tracer concentration of 177Lu within a voxel or VOI secondary to system calibration with a NISTtraceable Selenium 75-point source of known concentration, along with CT attenuation and scatter correction. Tracer concentration within an individual voxel or VOI defined from sequential SPECT/CT studies is used to generate accurate time activity curves for dosimetry calculation.
In the present study, the renal dose (left kidney: 3.73 Gy, right kidney: 3.79 Gy by MIRD) is comparable between MIRD and voxel-based dosimetric methods. The tumor dose measured in the largest liver metastases (22.4 Gy MIRD) is significantly higher than renal dose, as is expected based on the tracer uptake and clearance differences between the tumor and renal cortex.
An accurate estimation of renal dose is key in the determination of the maximum allowable number of 177Lu DOTATATE therapy cycles based on the accepted cumulative renal dose threshold of 23 Gy or a higher threshold of 40 Gy in patients with good renal function and without any risk factors like hypertension or diabetes. In a recent study, hybrid dosimetry (3 planar with single SPECT/CT) was performed on 51 patients with metastatic NET undergoing multiple therapies with 177Lu DOTATATE (7.4 GBq per therapy cycle).2 Most patients were treated based on a renal dose of 23 Gy with a median number of therapy cycles being 5 (range 3–7). However, 5 patients with good renal function without any other risk factors were treated up to a threshold of 40 Gy for cumulative renal dose and the median number of therapies in this group was 7 (range 5–8). Significant renal toxicity with glomerular filtration rate (GFR) < 30 ml/min/1.73 m2 was not seen in any patient. The absence of significant renal toxicity in patients treated with multiple therapies irrespective of their initial renal functional status is encouraging for offering these patients multiple therapies. However, the decrease in GFR with progressive number of therapies was higher in the group with lower initial GFR levels. Thus, repeated monitoring of renal status and accurate renal dosimetry following every therapy cycle is key to ensuring absence of significant long term renal impairment in these patients, especially those with associated hypertension or diabetes.
Since the patient had undergone 2 previous therapies with similar levels of renal doses and the current therapy cycle delivers a total renal absorbed dose of more than 3.5 Gy, planning of additional therapies needs to be based on correct assessment of total renal dose threshold for this patient. In view of normal renal function, normal renal cortical thickness on CT and low initial uptake and fast clearance of tracer from the renal cortex, a higher threshold of 40 Gy for cumulative absorbed renal dose for this patient is justified. Additional therapies with dosimetry for each therapy cycle can therefore be advocated, which may further improve tumor control. In view of the large tumor burden and high tracer avidity of the tumor, the amount of circulating tracer available for the kidney will be limited thereby reducing the risk of renal toxicity (tumor sink effect). However, large tumor burden is an adverse prognostic indicator irrespective of therapy type and somatostatin receptor avidity of the tumor and the objective of repeated radionuclide therapy would be primarily to decrease hormone release related symptoms, tumor mass effects, and improve quality of life.