On May 20, 2020, a new radiopharmaceutical agent was approved by the US Food and Drug Administration (FDA) for use with PET imaging: Fluoroestradiol F 18 (FES). Known in the US as Cerianna™, the radiopharmaceutical is an estrogen analog (16α-[18F]-fluoro-17β-estradiol) and the first F-18 PET imaging agent to be indicated for the detection of estrogen receptor (ER)-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer (MBC).1,2
Illstrations provided by Zionexa SAS
Cerianna was developed by Zionexa SAS (Paris, France) and is exclusively manufactured and distributed throughout the United States by PETNET Solutions, Inc., a Siemens Healthineers company (Knoxville, TN, USA). “Cerianna’s approval is a huge milestone for patients with recurrent and metastatic breast cancer,” says Peter Webner, CEO of Zionexa USA (New York, NY, USA). “It will soon be a tool available to help provide oncologists with a picture of the estrogen receptor expression. We can now image the whole body and provide information on ER status in many lesions, not just a single-biopsied lesion,” he declares.
Determining ER status in MBC
ER status is important in breast cancer for risk assessment and predicting response to therapy. Both the US National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) recommend ER testing of lesions in any primary or newly metastatic breast cancer.3,4
Approximately 75 percent of all breast cancers in women and 99 percent in men are ER-positive,5,6 and almost all these patients will be candidates for endocrine therapies including selective estrogen receptor modulators (SERMs), selective estrogen receptor degraders (SERDs), aromatase inhibitors, and cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors. The outlook for MBC patients has changed tremendously with the introduction of these therapies, Webner notes. Between 1985 and 2016, median overall survival in women with MBC improved from 13 months to 33 months and 5-year survival increased from 10 percent to 27 percent.7
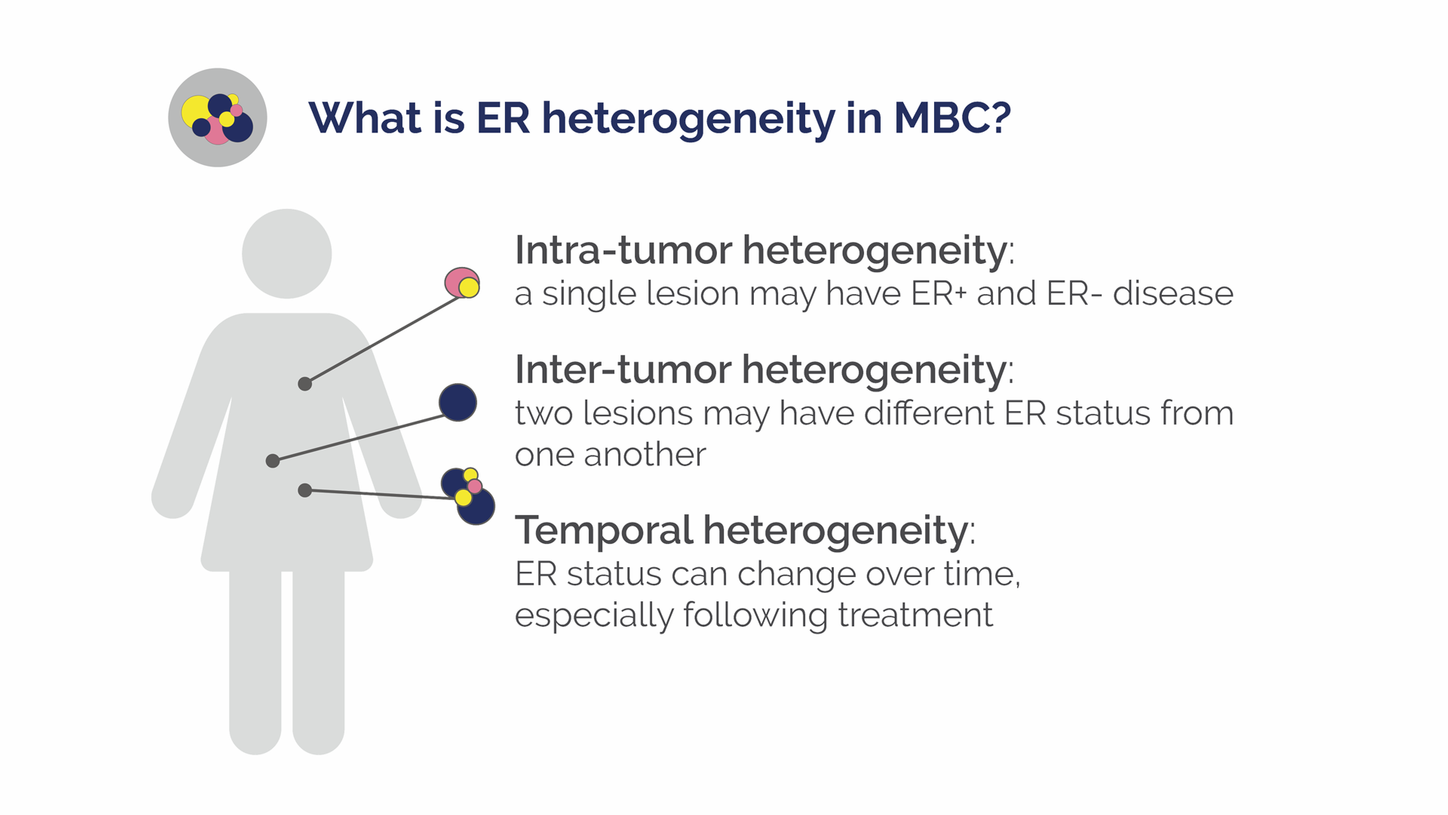
Despite the utility of endocrine therapy, ER discordance and inter-tumor heterogeneity likely contribute to low treatment response rates, Webner explains. Discordance in ER expression between the primary tumor and metastases occurs in about 20 percent of breast cancer patients.8
ER status may differ between metastatic lesions, and cancers that switch to low ER expression (around 20 percent) often have characteristics more similar to ER-negative cancers. These types of cancers are unlikely to respond to hormonally driven therapy, which has clinical and economic consequences, explains Yann Bouvet, PhD, CSO, at Zionexa SAS.
To date, the gold standard for determining ER status in MBC is immuno-histochemistry (IHC) on tissue biopsies from the primary tumor and usually a single metastasis, although most MBC patients have many metastases, most frequently in bone and lung. Obtaining tissue by biopsy can be particularly difficult at these sites and IHC is not used consistently in MBC patients, even if recommended by guidelines like those of the NCCN, Bouvet says. As a result, he maintains, treatment decisions are often based on incomplete and imperfect information. Another disadvantage of biopsy is that it is an invasive and painful exam, he notes.
FES-PET versus Biopsy IHC
The use of FES in PET imaging (FES-PET) has many advantages for the clinician and patient, Bouvet stresses. The FES imaging agent is administered by intravenous injection and provides results within 24 hours of the exam, compared to two weeks with IHC. Importantly, unlike IHC, FES-PET can assess ER expression of every tumor in the body simultaneously, providing what Webner calls, “an ER status map of the whole body.” FES-PET/CT can also distinguish between ER-positive and -negative lesions, which cannot be done with standard imaging.
FDA approval for Cerianna was based primarily on trial data that confirmed the diagnostic accuracy of FES-PET versus IHC9 and the correlation of low FES uptake with lack of ER expression.10 Analysis of ER status determined by FES-PET versus biopsy IHC showed an overall sensitivity and specificity of 83 percent on metastatic lesions and primary tumor.11
FES-PET is associated with acceptable levels of radiation exposure and a high safety profile with the potential for injection site pain and dysgeusia as the main side effects, occurring in less than one percent of patients. The primary consideration for FES-PET is the need for prior washout of drugs like tamoxifen or fulvestrant, which block the estrogen receptor and would impact the uptake of FES, Bouvet explains.

FES-PET-driven personalized medicine
“Our goal is to be able to help identify the patients that will best benefit from therapy,” Webner stresses. “With FES-PET we can identify patients who have estrogen-driven disease versus the ones who don’t, or the ones who have mixed disease. Then the clinician has actionable data with which they can make a therapeutic choice that should help them extend the patient’s survival and hopefully give them a better outcome.” When the options involve expensive drugs, the choice can also be cost effective,” Webner adds. For example, patients with 100 percent FES positivity appear to benefit most from treatment with CDK4/6 inhibitors12 and, “since they are not indicated for ER-negative disease, there’s clear financial benefit in having the patient go on to an alternate therapy that will give better outcomes,” Webner explains.
Zionexa aims to extend the indication for Cerianna by supporting what was observed in small populations during clinical trials to use it for early response to endocrine therapy. The IMP ACT-MBC (IMaging PAtients for Cancer Drug selecTion - Metastatic Breast Cancer) trial is looking at the clinical utility of experimental PET scans, including FES, in the setting of MBC at first presentation, and initial results appear promising.13 Another multicenter clinical trial being carried out by the ECOG-ACRIN Cancer Research Group is evaluating the predictive value of FES in patients with newly diagnosed breast cancer.
“New biomarkers just don’t come around that often and this one, in particular, is highly attractive in the market today.”
New Era for an Old Concept
FES was first synthesized over 30 years ago14 and has since been studied in breast cancer imaging worldwide, Webner recalls. The original formulation was stable for only 4-6 hours, and could only be produced in small batches. Zionexa was able to develop an industrial process and subsequently obtain a formulation with high-specific activity and an expiry period of 10-12 hours. The company was granted a patent for its formulation and process in July 2020.
“F-18 isotopes have a half-life of 110 minutes, so partnering with a world class organization like PETNET Solutions will be key to timely regional manufacturing and distribution throughout the country,” Webner emphasizes. Barry Scott, CEO of PETNET Solutions, explains that with a network of 43 cyclotron-equipped pharmacies across the United States, PETNET Solutions can reach a broad spectrum of population in the nation, ensuring that hospitals and imaging centers have access to novel biomarkers like Cerianna.
New products are always challenging to launch, Webner observes. “Changing the way physicians practice is not a quick process and we need to work with oncologists to help them identify the correct patient profiles for this exam and with imagers to ensure they provide actionable information to the referring physicians,” he states. “Since FES-PET is a type of receptor imaging, interpreting a Cerianna scan is different from most other PET tracers currently in use. An important step will be to have FES-PET included in national breast cancer management guidelines, which are key in breast cancer care,” Webner adds.
Based on PETNET Solutions’ commercial experience, Scott is optimistic about the future of Cerianna. “New biomarkers just don’t come around that often and this one, in particular, is highly attractive in the market today,” he declares. A phased US roll-out of Cerianna is set to begin by early 2021.
About the author
Linda Brookes, MSc, is a freelance medical writer and editor, dividing her time between London and New York, working for a variety of clients in the healthcare and pharmaceutical fields.
Cerianna™ (18F-FES)
Indications and Usage
- 18F-FES is a radioactive diagnostic agent indicated for PET imaging.
- 18F-FES is indicated for the detection of ER-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer.
Limitations of Use
- Tissue biopsy should be used to confirm recurrence of breast cancer and to verify ER status by pathology. 18F-FES is not useful for imaging other receptors, such as HER2 and PR.
Important Safety Information
Adverse Reactions – Reported adverse reactions include: injection site pain and dysgeusia.
Radiation Risks – Ensure safe drug handling and patient preparation procedures to protect patients and health care providers from unintentional radiation exposure.
Risk of Misdiagnosis - Do not use CERIANNA in lieu of biopsy when biopsy is indicated in patients with recurrent or metastatic breast cancer.
Contraindications – None.
Use in Specific Populations – Lactation: interrupt breastfeeding.
Advise a lactating woman to avoid breastfeeding for 4 hours after CERIANNA administration.
To report SUSPECTED ADVERSE REACTIONS, contact Zionexa US Corp at +1.844.946.6392 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Dosage and Administration
Dosage form and strengths
- Injection: clear, colorless solution in a multiple-dose vial containing 148 MBq/mL to 3,700 MBq/mL (4 mCi/mL to 100 mCi/mL) of Cerianna at end of synthesis.
Patient preparation
- Drink water to ensure adequate hydration prior to administration of 18F-FES
- Continue drinking and voiding frequently during the first hours following administration to reduce radiation exposure
Dosage and administration
- Activity recommended is 222 MBq (6 mCi), with a range of 111 MBq to 222 MBq (3 mCi to 6 mCi)
- Administration : single IV injection of 10 mL or less over 1 to 2 minutes
- Use aseptic technique and radiation shielding when withdrawing and administering FES.
- Visually inspect the radiopharmaceutical solution
- FES may be diluted with 0.9% Sodium Chloride Injection, USP
- Assay the dose in a suitable dose calibrator prior to administration
- Post administration
- Follow FES injection with an IV flush of 0.9% Sodium Chloride injection, USP
Safety of 18F-FES
Safety was determined from 1,207 patients with breast cancer receiving at least one Fluoroestradiol F18 administration
Age range = 21-91 years
- 98% were women
- 76% were post-menopausal
Safety profile was based on clinical studies + NCI Investigator’s brochure:
- No serious adverse events
- Adverse events with <1% frequency
- Injection site pain
- Dysgeusia



