History
A 57-year-old male with history of difficulty in swallowing along with weight loss of more than 5 kg in one month was investigated with upper gastrointestinal endoscopy. Endoscopy revealed a mass in the lower third of the esophagus with irregular thickening of mucosa protruding into the esophageal lumen, causing narrowing such that the endoscope could not pass beyond the esophageal lesion. Histopathology from the mucosal biopsy demonstrated a squamous cell carcinoma.
The patient underwent a CT scan for initial evaluation, as well as CT simulation for radiation therapy. CT showed an irregular thickening of the esophagus at the lower third with protrusion of the mass into the esophageal lumen, causing narrowing. Loco-regional lymph node enlargement was not detected on the CT scan.
CT simulation did not clearly delineate the tumor margins from the normal esophagus and adjacent structures, limiting accurate contouring for radiation therapy. In this context the patient was referred for Fludeoxyglucose F 18 injection (18F FDG) PET/CT for delineation of true tumor extent and the presence of local and distant metastases.
An 18F FDG PET/CT study was performed on a Biograph™ PET/CT system fitted with a flat table top and positioning laser. The scan was acquired one hour following an IV injection of 10 mCi of 18F FDG. Following the initial diagnostic CT acquisition, the PET study was acquired at 3 minutes per bed for 7 bed positions.
Findings
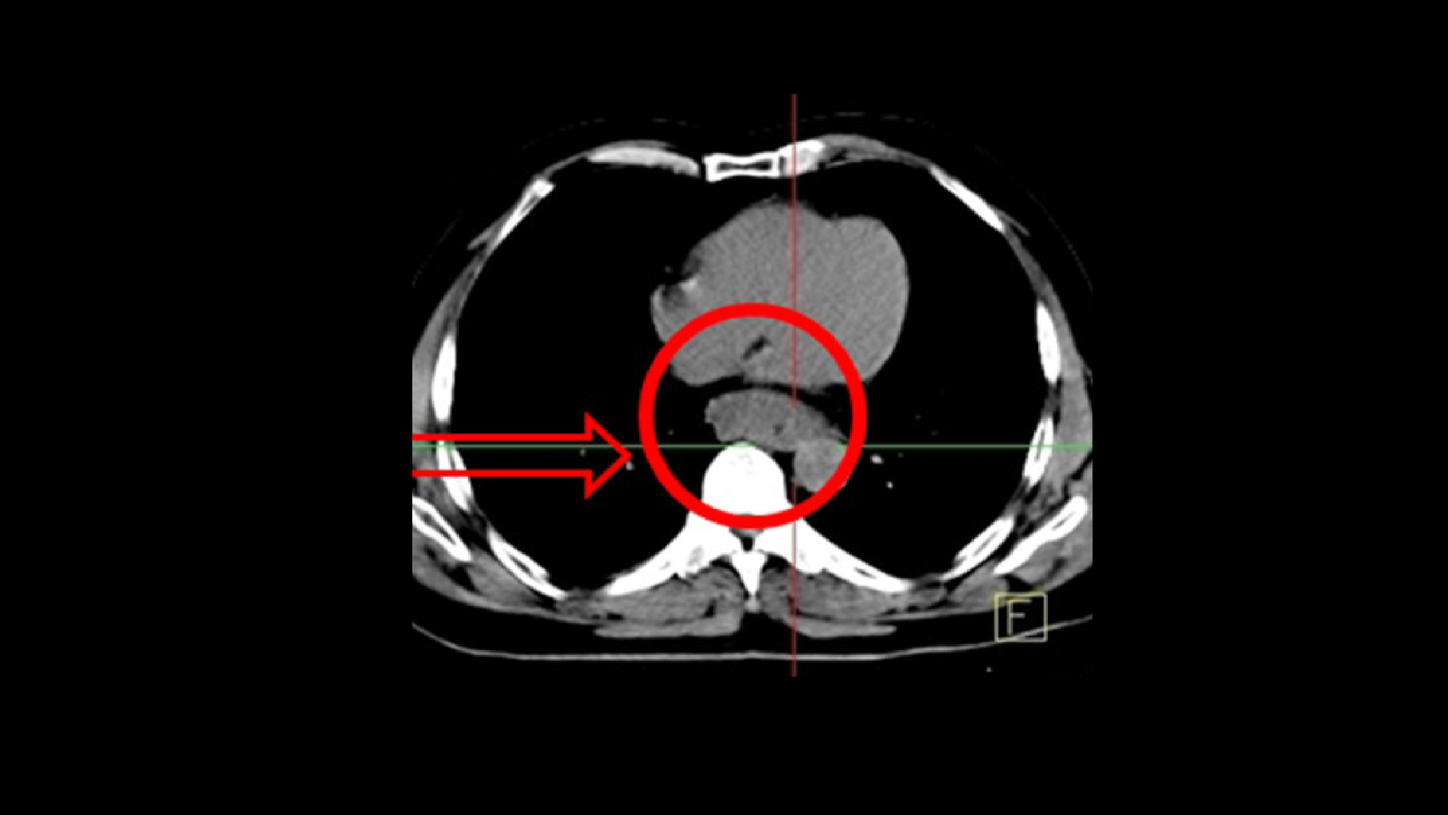
As shown in Figure 3, PET/CT sharply delineated hypermetabolic tumor margins in the lower third of esophagus (size 3 x 4, 6 x 6 cm; SUVmax 10.4) without evidence of lymph node or distant metastases. The PET/CT, however, showed focal hypermetabolism in the larynx. Histopathology of the right pyriform sinus lesion also demonstrated a squamous cell carcinoma suggestive of a second malignancy.
The patient was recommended for radiation therapy with concurrent chemotherapy with the esophageal tumor irradiated initially. Chemotherapy included a Docetaxel and Cisplatin regimen for 3 periods (21-day cycles). The chemotherapy regime included an intravenous administration of Docetaxel 75 mg/m2/day along with Cisplatin 75 mg/m2/day. Radiation therapy planning for the esophageal lesion was performed based on the 18F FDG PET/CT images with gross tumor volume (GTV) incorporating the FDG-avid tumor zone. A total GTV dose of 50.4 Gy was prescribed to be delivered at the rate of 1.8 Gy/day.
The patient was reassessed on the completion of 3 chemotherapy cycles combined with radiation therapy. The patient showed a significant improvement in the ability to swallow. A follow up 18F FDG PET/CT was performed to assess disease status on the completion of chemoradiation therapy. The PET/CT acquisition protocol was similar to the initial staging study. The post chemoradiation PET/CT showed a gross decrease in the esophageal tumor mass with substantial decrease of lesion hypermetabolism and SUVmax. The laryngeal mass showed a slight decrease in size, as well as lesional hypermetabolism and SUVmax following chemotherapy.
The patient underwent a follow-up gastroscopy to evaluate the esophageal lesion post chemoradiation. The endoscopy confirmed a complete regression of the esophageal tumor with patency of the esophageal lumen restored (Figure 6). Radiation therapy to the laryngeal lesion was subsequently planned based on post chemotherapy PET/CT. GTV was delineated based on the hypermetabolic laryngeal tumor seen on PET/CT and 70 Gy was prescribed to the GTV with 50 Gy delivered to the cervical neck nodes. A standard fractionation regime was used (1.4 Gy/fraction to CTV, including bilateral lymph nodes). The patient developed radiation-related toxicity like oral ulcers, fever and leucopenia, which were adequately managed with antibiotics and parenteral nutrition.
On the completion of laryngeal radiation therapy, the patient underwent a laryngoscopy which showed a disappearance of the laryngeal mass (Figure 8).
A follow-up 18F FDG PET/CT was performed using similar acquisition parameters as the previous studies. PET/CT showed a disappearance of the laryngeal mass following radiation therapy, confirming the findings ofthe laryngoscopy.
The patient was subsequently clinically followed up and showed progressive improvement with increase in body weight, absence of swallowing difficulties and freedom from any respiratory distress.
Comments
This case demonstrates the value of 18F FDG high-resolution PET/CT in initial staging and radiation therapy planning for both esophageal and laryngeal cancer and also illustrates an uncommon condition of a second primary cancer (laryngeal squamous cell carcinoma) associated with a primary esophageal carcinoma. 18F FDG PET/CT enabled an accurate delineation of tumor extent for precise radiation therapy planning, allowing adequate tumor dose to be delivered to a hypermetabolic viable tumor while sparing surrounding normal tissue and thereby, reducing radiation-induced toxicity. Sequential 18F FDG PET/CT helped to ascertain tumor response and to plan subsequent therapy. As evident from the sequential images, the esophageal tumor showed a complete regression following initial chemotherapy and radiation to the esophageal GTV. However, the laryngeal tumor showed a minor response to initial chemotherapy, and second-phase radiation to the laryngeal tumor was planned on the post-chemotherapy PET/CT, enabling precise GTV delineation. This enabled a dose of 70 Gy to be delivered to the tumor with only mild toxicity (oral ulcers), which were easily managed.
Conclusion
18F FDG PET/CT enables detection ofsecond primary tumor in the larynx ina patient with esophageal carcinoma. Chemoradiation therapy with accurate targeting of metabolically active tumor volume demonstrated on PET/CT was instrumental in achieving tumorfree status.