Duyarlılık ve Özgüllük
- Test duyarlılığı, testin hastalığı olan hastaları doğru bir şekilde belirleme yeteneğini gösterir. Bir testin duyarlılığı, gerçek pozitif oran olarak da bilinir. Bir tanı testi, tüm pozitif sonuçların %100'ünü doğru bir şekilde tanımladıysa, mümkün olduğu kadar hassas olacaktır.
- Test özgüllüğü, testin hastalığı olmayan hastaları doğru bir şekilde belirleme yeteneğini gösterir. Bir test, hastalığı olmayan tüm insanları doğru bir şekilde negatif olarak tanımlarsa, mümkün olduğu kadar spesifik olur.
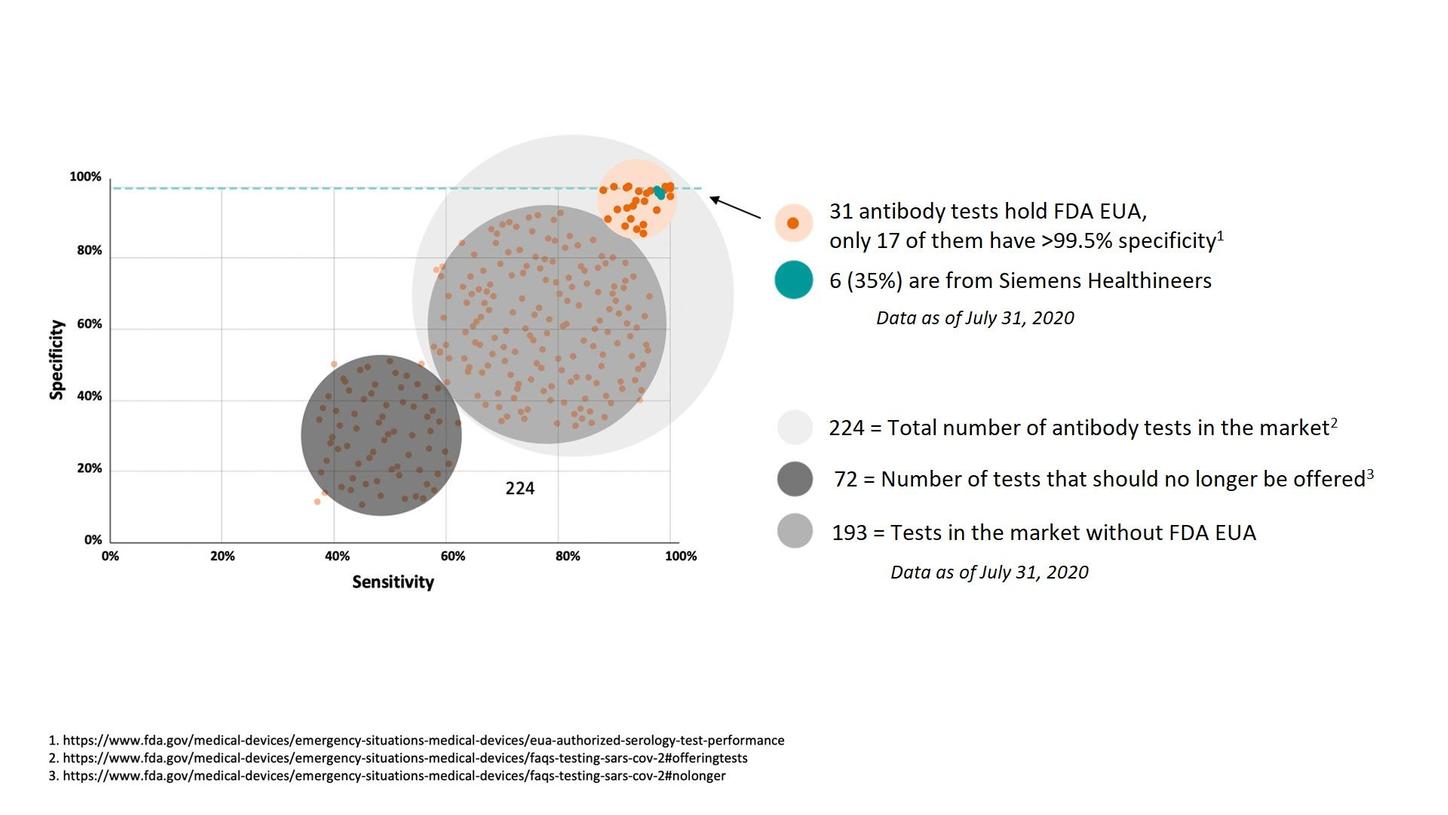
COVID-19 testinde özgüllük
SARS-CoV-2 virüsüne karşı antikorları tespit ettiğini iddia eden çok sayıda test vardır; sadece birkaçı son derece doğrudur:
Yüksek düzeyde spesifik antikor testleri, düşük hastalık prevalansında bile iyi performansı nasıl destekler?
Biri %5 hastalık prevalansı, diğeri %10 prevalansı olan iki şehirde neler olduğuna bakalım.
Popülasyon, %99,8'lik daha yüksek özgüllüğe sahip bir tahlille test edildiğinde ne olur?
Son derece spesifik bir test, hatalı sonuçları en aza indirir. Daha yüksek bir hastalık prevalansı ile daha az insan yanlış sonuçlarla karşılaşacaktır.
Özgüllük %96'ya düşürüldüğünde ne olur?
%96'lık bir özgüllük yüksek görünebilir, ancak yüzde 3 ila 4 kadar küçük bir fark, test sonuçlarında çarpıcı değişiklikler yaratabilir.
The model above assumes assay sensitivity of 100%