FlowMotion AIMoving the standard to personal care
FlowMotion™ AI introduces continuous bed motion for standardized and personalized acquisitions with the click of a button.
Features & Benefits

FlowMotion AI gives you the ability to develop and save protocols based on clinical indication—incorporating motion management and high-resolution imaging into clinical routine, the result is:
- More reliable visualization of disease and ensured reproducibility
- Simple and precise range planning enables personalization of scans to each organ in order to meet individual patient needs
- Elimination of CT over-scanning and the associated radiation exposure
- Sense of continuous progression also provides a more comfortable exam experience for patients
With the addition of our deep learning AI algorithm, Automated Landmarking and Parsing of Human Anatomy (ALPHA) technology, you can simply load the disease-specific protocol and the let scanner define your protocol ranges. This creates a new level of standardization and personalization where every patient exam is set up using AI, independent of the user.
Standardizing imaging protocols based on indication. Personalizing scans based on anatomy.

Faster workflow

More personalized scan

Reproducible results from any operator

Simple and precise range planning that helps avoid cut-offs or over radiation
What the experts are saying
"With FlowMotion AI we've noticed it speed things up, and It makes a difference in the workflow, especially for patients who, for different reasons, are anxious to get off the scanner as quickly as possible."

Katie Morris
The Royal Marsden NHS Foundation Trust
London, England, UK

Continuous bed motion
FlowMotion is synonymous with continuous bed motion (CBM), an innovation that relies on advances in conventional patient bed technology, scanner acquisition electronics, and algorithms for processing scanner data.
Patient bed
- Magnetically driven
- Sub-millimeter (< 0.25 mm) positioning and smooth acceleration
- Speeds ranging from 0.1 to 200 mm/s
- Minimized vertical deflection
Redesigned acquisition electronics
- Custom architecture processes and stores precise bed positioning along with raw PET data
Dynamic data processing algorithms
- PET data is continuously normalized during the acquisition to account for the corresponding motion of each line of response (LOR)
ALPHA
ALPHA technology is a unique, deep learning AI algorithm that detects specific anatomical landmarks. With FlowMotion AI, landmarks associated with the top of the head, beneath-the-eye orbits, the clavicle, the aortic arch, the adrenal gland, the mid-thigh, and the tip of the toes are obtained from the CT topogram and used to establish PET scan ranges routinely used within clinical workflows.
By integrating continuous-bed-motion technology with ALPHA landmarking, FlowMotion AI renders PET planning less complicated for the user, more efficient, and more repeatable than manual scan planning.
Clinical Use
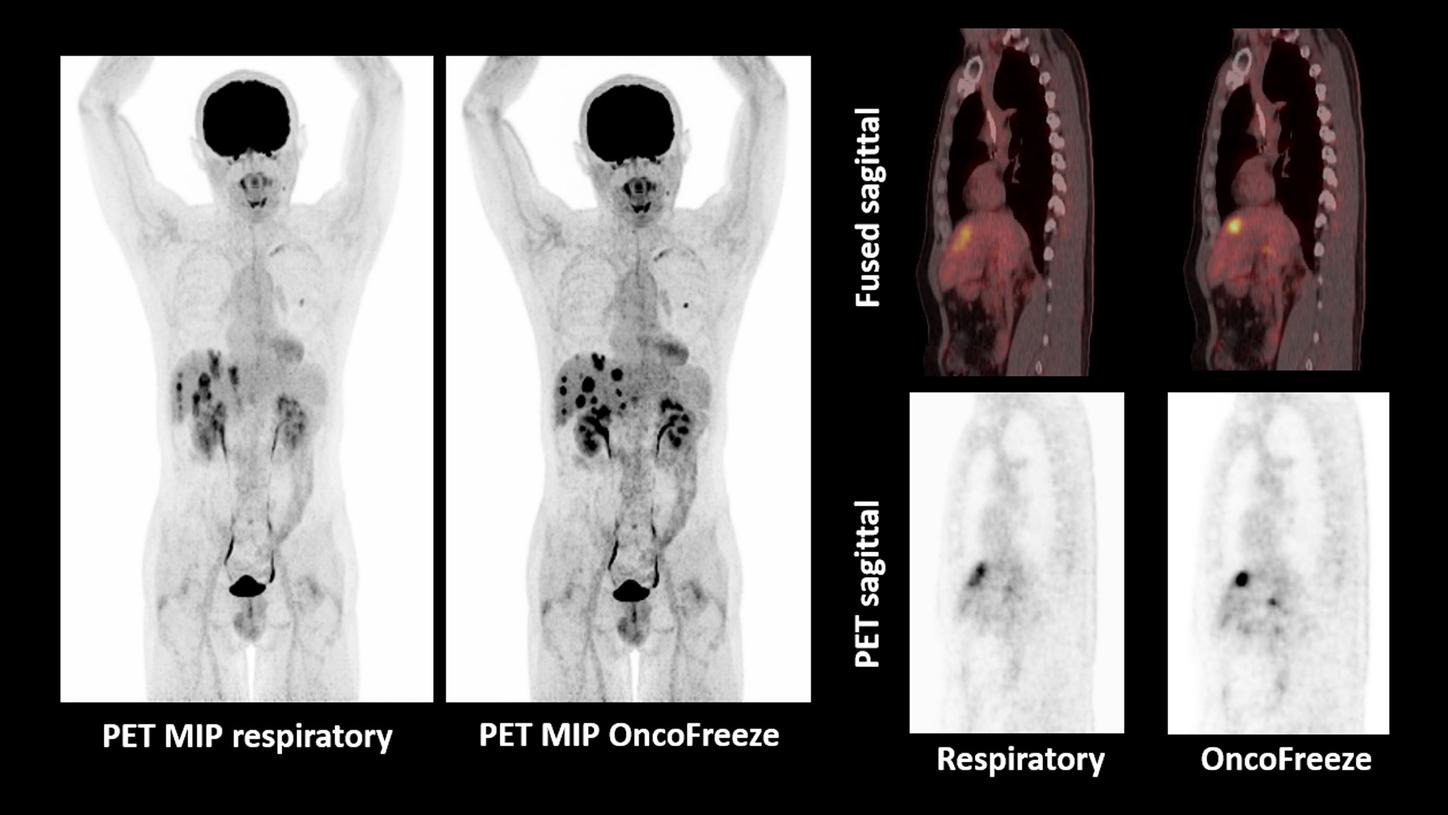
OncoFreeze improves visualization of liver metastases with respiratory motion management
- Multiple hypermetabolic liver metastases in a patient with operated colorectal carcinoma with severe respiratory-motion-related blurring seen in non-gated PET
- Respiratory motion management with OncoFreeze helps show sharp delineation and improved contrast in liver metastases by eliminating respiratory-motion-related blurring
- Motion-frozen images obtained without additional scan time requirement due to utilization of all acquired data
PET – Biograph Vision™
Scan acquisition
440 x 440 matrix, PSF+ToF 3i5s
All-pass filter
Scan time
FlowMotion continuous bed motion
Whole-body single zone 0.9 mm/s
20.6 min total scan time
Injected dose
Fludeoxyglucose F 18 (18F FDG) Injection
5.5 mCi (203 MBq)
CT
Scan parameters
120 kV/10 ref mAs

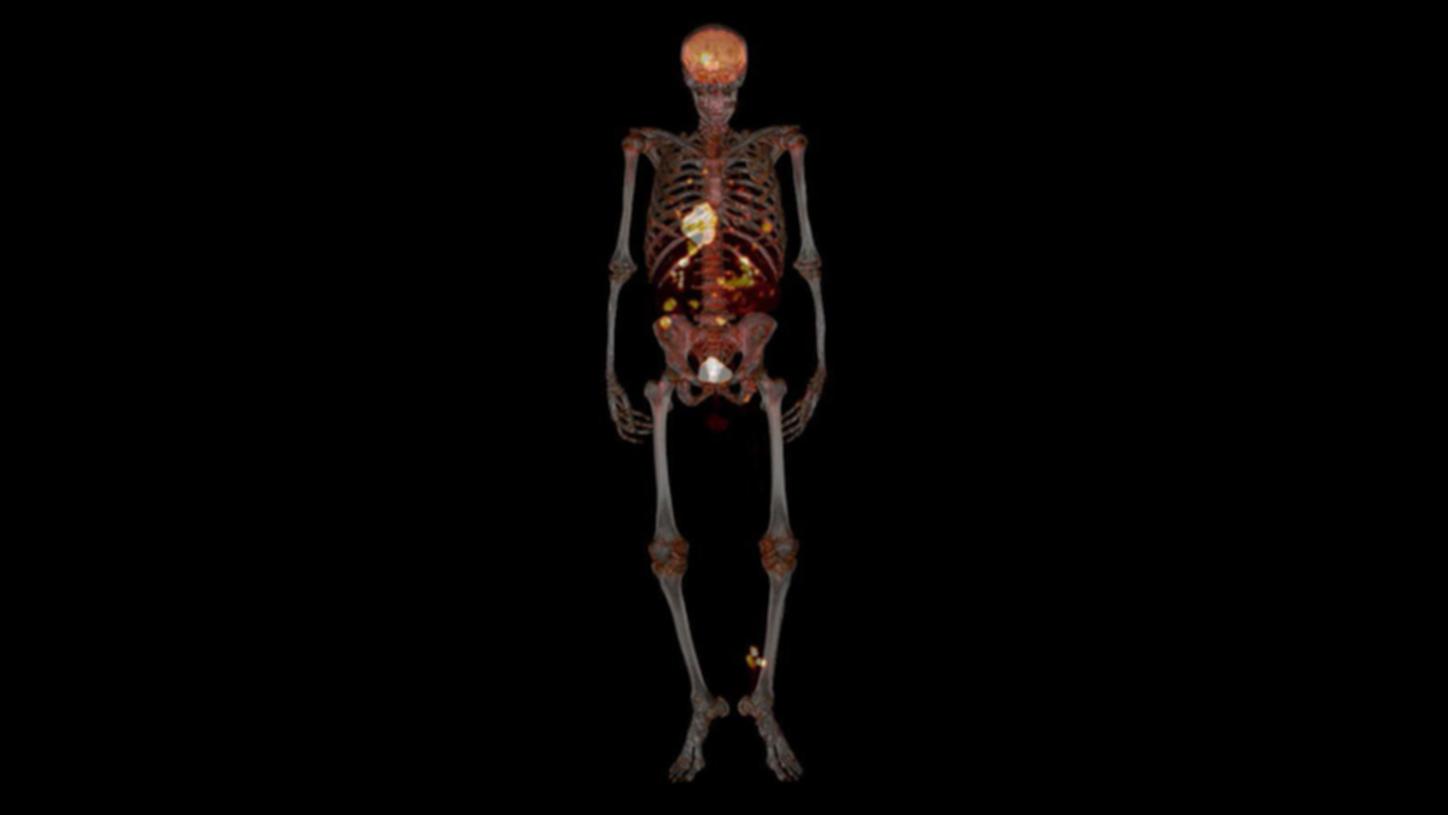
Skull metastases in a patient with lung carcinoma delineated by PET/CT and FlowMotion
Clinical detail
A patient presented with lung carcinoma with hilar and supraclavicular metastases. A PET/CT was performed on Biograph mCT Flow™ with FlowMotion to rule out distant metastases.
The study shows small metastatic foci in the skull corresponding to a lytic lesion seen on CT. The small lesion with low tracer uptake is well- visualized due to uniform axial noise sensitivity even at the edges of the acquisition range, made possible by FlowMotion acquisition.
Examination protocol
Biograph mCT Flow 64, 6.6 mCi 60-minute post-injection delay CT: 120 kV 50 eff. mAs.
Whole-body dynamic PET/CT with FlowMotion enables the visualization of distribution over the entire body
Examination protocol
Biograph mCT Flow 64, 6.9 mCi (254 MBq) 18F FDG injection[a], whole-body dynamic acquisition, 24 frames, CT: 100 kV, 68 eff mAs.

OncoFreeze improves visualization of liver metastases with respiratory motion management
- Multiple hypermetabolic liver metastases in a patient with operated colorectal carcinoma with severe respiratory-motion-related blurring seen in non-gated PET
- Respiratory motion management with OncoFreeze helps show sharp delineation and improved contrast in liver metastases by eliminating respiratory-motion-related blurring
- Motion-frozen images obtained without additional scan time requirement due to utilization of all acquired data
PET – Biograph Vision™
Scan acquisition
440 x 440 matrix, PSF+ToF 3i5s
All-pass filter
Scan time
FlowMotion continuous bed motion
Whole-body single zone 0.9 mm/s
20.6 min total scan time
Injected dose
Fludeoxyglucose F 18 (18F FDG) Injection
5.5 mCi (203 MBq)
CT
Scan parameters
120 kV/10 ref mAs

Skull metastases in a patient with lung carcinoma delineated by PET/CT and FlowMotion
Clinical detail
A patient presented with lung carcinoma with hilar and supraclavicular metastases. A PET/CT was performed on Biograph mCT Flow™ with FlowMotion to rule out distant metastases.
The study shows small metastatic foci in the skull corresponding to a lytic lesion seen on CT. The small lesion with low tracer uptake is well- visualized due to uniform axial noise sensitivity even at the edges of the acquisition range, made possible by FlowMotion acquisition.
Examination protocol
Biograph mCT Flow 64, 6.6 mCi 60-minute post-injection delay CT: 120 kV 50 eff. mAs.
Whole-body dynamic PET/CT with FlowMotion enables the visualization of distribution over the entire body
Examination protocol
Biograph mCT Flow 64, 6.9 mCi (254 MBq) 18F FDG injection[a], whole-body dynamic acquisition, 24 frames, CT: 100 kV, 68 eff mAs.

OncoFreeze improves visualization of liver metastases with respiratory motion management
- Multiple hypermetabolic liver metastases in a patient with operated colorectal carcinoma with severe respiratory-motion-related blurring seen in non-gated PET
- Respiratory motion management with OncoFreeze helps show sharp delineation and improved contrast in liver metastases by eliminating respiratory-motion-related blurring
- Motion-frozen images obtained without additional scan time requirement due to utilization of all acquired data
PET – Biograph Vision™
Scan acquisition
440 x 440 matrix, PSF+ToF 3i5s
All-pass filter
Scan time
FlowMotion continuous bed motion
Whole-body single zone 0.9 mm/s
20.6 min total scan time
Injected dose
Fludeoxyglucose F 18 (18F FDG) Injection
5.5 mCi (203 MBq)
CT
Scan parameters
120 kV/10 ref mAs


Technical Specifications
General Requirements
System
- Biograph Vision PET/CT
- Biograph Vision Quadra™ PET/CT
- Biograph mCT PET/CT
- Biograph Horizon PET/CT
Minimum Software Version
Biograph Horizon must run SW PETsyngo VJ30 or higher
Biograph mCT and Biograph Vision must run SW PETsyngo VG80 or higher
Biograph Vision Quadra must run SW PETsyngo VR20 or higher
Other
PET respiratory gating or PET/CT respiratory gating option is required to enable this option.
Learn more about FlowMotion AI
The statements by Siemens Healthineers' customers described herein are based on results that were achieved in the customer's unique setting. Because there is no “typical” hospital or laboratory and many variables exist (e.g., hospital size, samples mix, case mix, level of IT and/or automation adoption) there can be no guarantee that other customers will achieve the same results.