History
Diagnosis
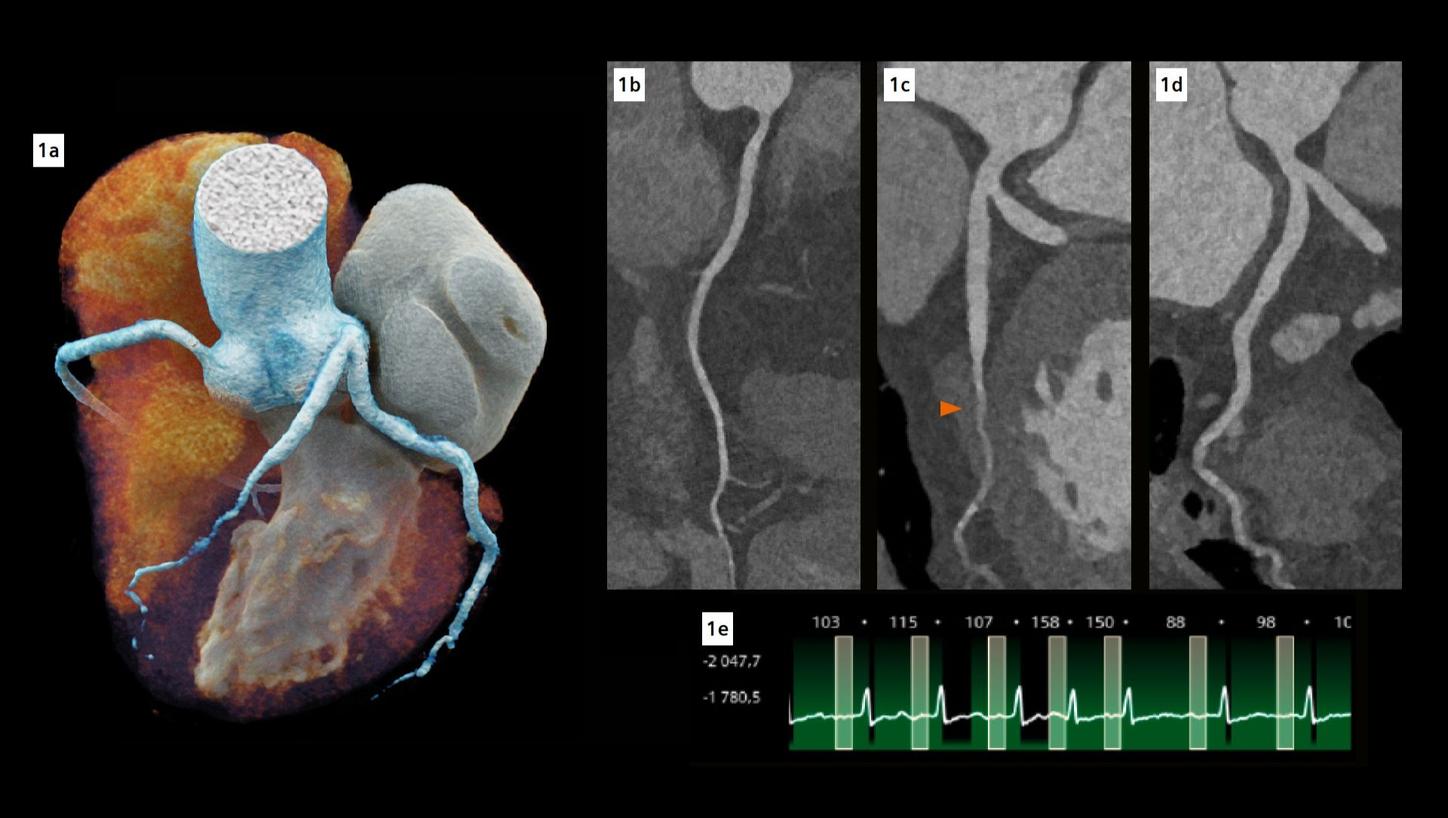
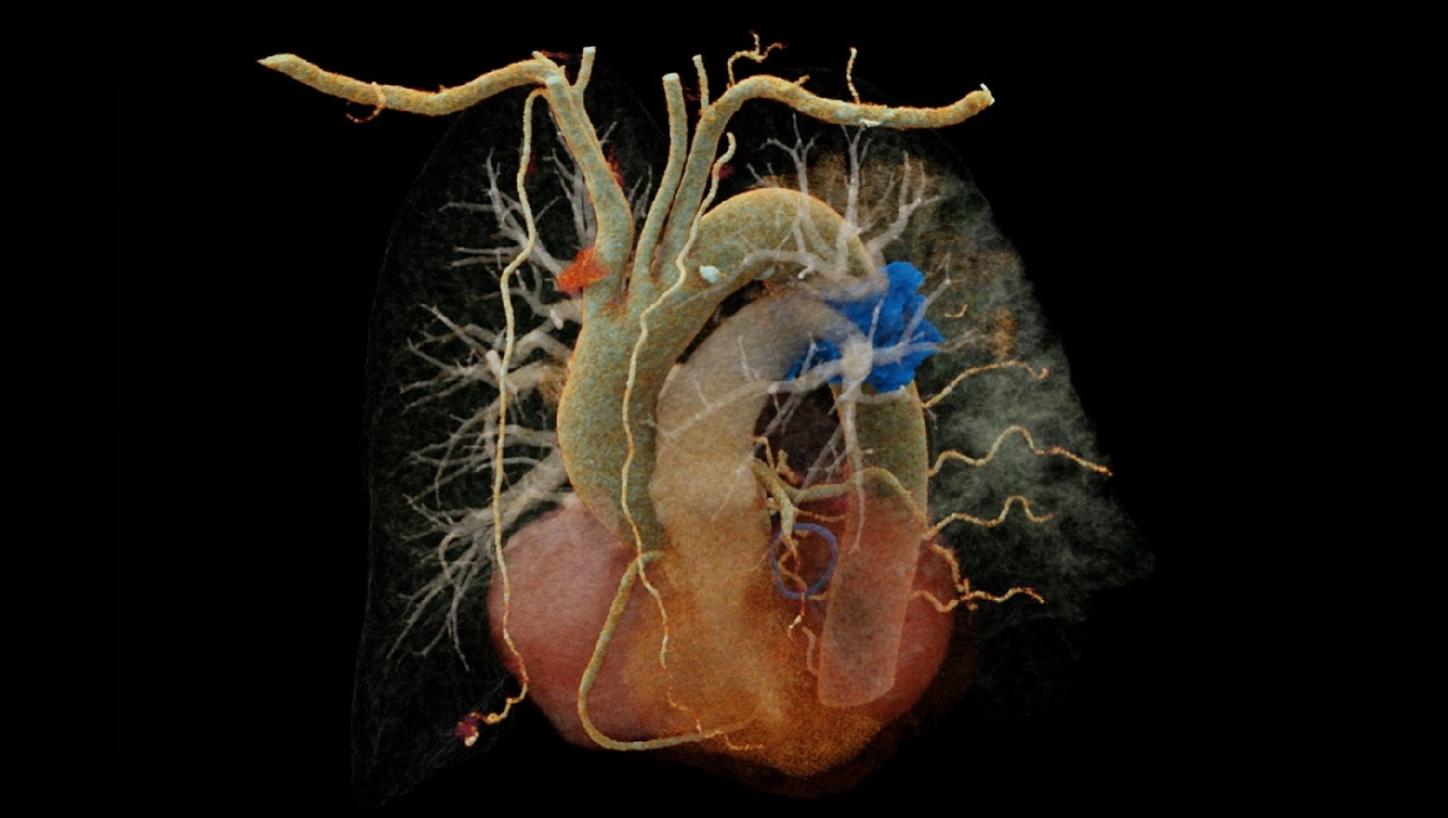
Fig. 1: Cinematic volume rendering technique (cVRT) image of the heart (a) and curved multiplanar reconstructions of the coronary arteries (b-d). (b) represents the right coronary artery, (c) represents the left main and left anterior descending (LAD) coronary artery, while (d) represents the left main and left circumflex coronary artery. The arrow indicates a complete myocardial bridge on the middle LAD. Note the excellent visualization of the coronary arteries despite high and irregular heart rate (e).
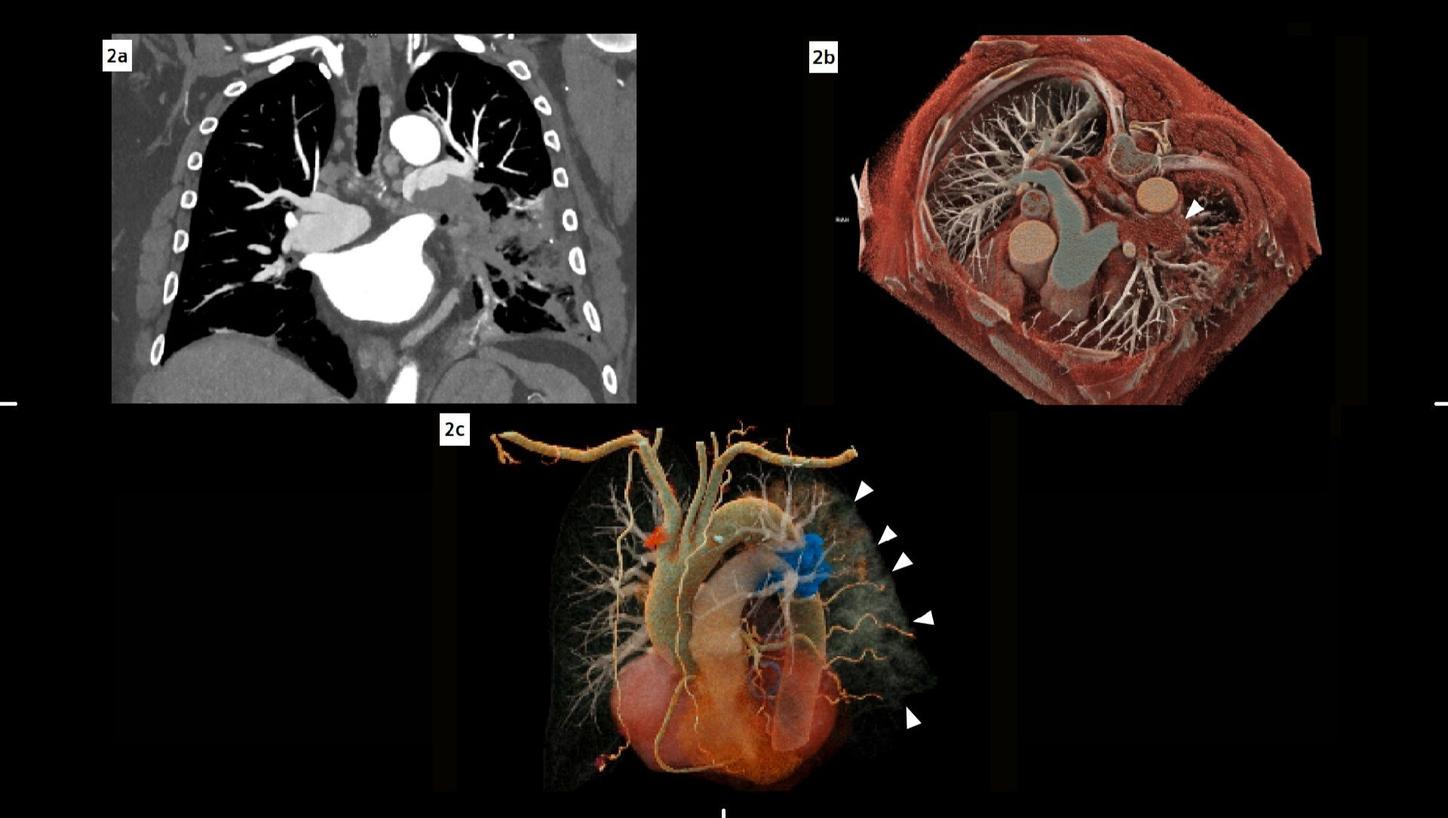
Fig. 2: Coronal maximum intensity projection (MIP) image of the pulmonary vasculature (a) demonstrates a filling defect in the lower part of the left pulmonary artery. Volume rendering technique (VRT) reconstructions (b, c) depict the pulmonary vessel arborization: In (b), a cross-sectional view, the filling defect is visible in the left pulmonary artery (arrow). In (c), an oblique view, the embolus is highlighted in blue, while the consolidations in the lung can also be appreciated (small arrows).
Comments
While the ability of triple-rule-out-CT (TRO-CT) to rule out disorders in all three vascular beds (pulmonary embolism, PE; aortic dissection, AD; and coronary artery disease, CAD) has been demonstrated [1], a gap remains in the literature regarding the clinical impact of TRO-CT. Araoz P.A. et al. found that, based on accepted clinical risk scores, 65% of their patient cohort warranted work-up for at least two of either PE, AD or CAD suggesting that a large subset of patients would benefit from a comprehensive test like TRO-CT [2], even though the mean radiation dose (23.8 ± 12 mSv; range: 3.7–84.1 mSv) and contrast media usage (144.6 ± 31.2 mL; range: 54-360 mL) varied widely. On the other hand, Takx et al. found that TRO-CT may reduce the length of stay and costs in ED in appropriate patients, but no difference in the diagnosis of PE, AD, or CAD was observed between TRO-CT versus standard-of-care strategies [3]. Currently, no major US or EU guidelines recommend routine TRO-CT as the initial ED test for undifferentiated acute chest pain, due to the limited contemporary comparative evidence. This case report serves as a reference, suggesting that PCD-CT may help revive the TRO-CT paradigm.
In this case, the targeted chest CTA enabled the use of a TRO-CT equivalent methodology. Therefore, the entire thoracic aorta was included in the scan range and timing was set to achieve optimal enhancement in the aorta and pulmonary arteries. Due to the high spatial resolution of the photon-counting detector, the coronary arteries could also be accurately evaluated.
The fine spatial detail (0.4 mm slice thickness) enabled an accurate evaluation of the coronary arteries to rule out severe CAD (as part of the differential diagnosis of chest pain) (see Figure 1). The use of advanced image reconstruction algorithms, such as ZeeFree, combined with the preview series function, resulted in only minor motion artifacts on the acquired scans, despite the high heart rate variability (Minimum: 86, Maximum: 158, average: 110). Detector-based quantum spectral imaging capabilities (lung analysis reconstruction) made it impressively fast to detect lung perfusion deficits caused by the PE and aided further therapy decision-making by down-grading disease severity. A fair dose length product (636 mGy*cm) was achieved during the chest CTA, utilizing the NAEOTOM Alpha.Prime CT scanner with an optimized protocol, serving as a true “one-stop-shop” TRO-CT examination.
Examination Protocol