Why Antibody Tests?
Antibody tests play an important role throughout the patient care pathway and are vital for the management and surveillance of the virus. They are critical in determining the full scope of the disease, combating the pandemic, and rebuilding public confidence.
Highly accurate antibody tests help inform clinical and public health decisions as we look towards safely opening our communities. Antibody tests can be used for the following:
- As an adjunct to PCR tests to aid in clinical assessment1,2
- To help determine prior exposure to the virus, by detecting antibodies that may neutralize the virus3,4
- To identify potential donors of convalescent plasma3-5
- For epidemiological purposes including establishing prevalence of disease in populations
- May help verify effectiveness of vaccines as they become available3,4
Specificity in COVID-19 testing
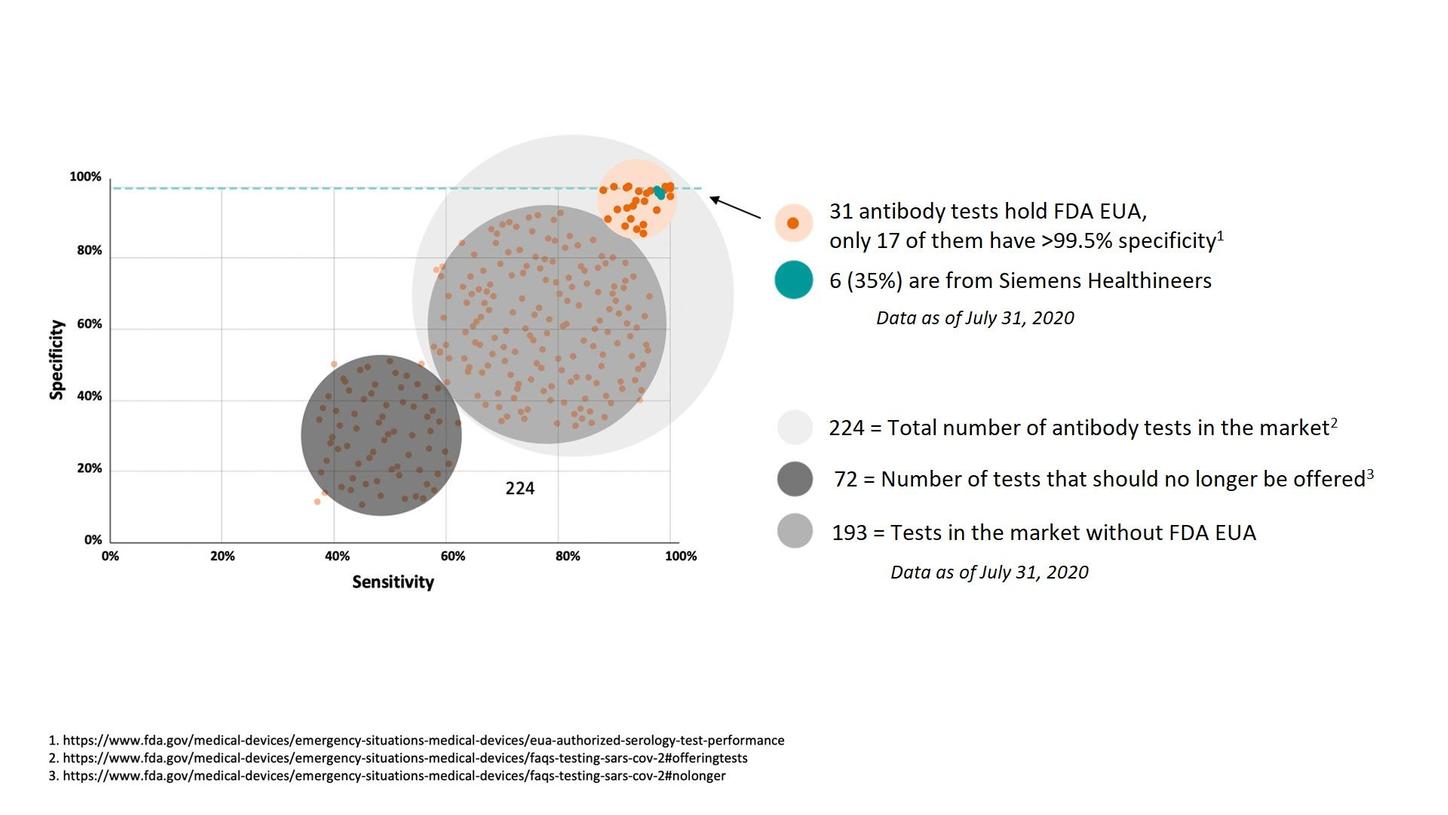
For SARS-CoV-2 antibody testing, the CDC suggests use of tests with a specificity ≥99.5% to minimize the potential for false-positive results.2
There are numerous tests that claim to detect antibodies to the SARS-CoV-2 virus; only a few are highly accurate:
Which antibody tests?
When an individual is infected with the SARS-CoV-2 virus, unique antibodies will develop at different stages of the infection.
SARS-CoV-2 Total antibody tests detect both antibodies (IgM and IgG) that are present during active infection or early during the immune response. SARS-CoV-2 IgG antibody tests specifically detect IgG antibodies that persist and are the basis for an individual’s longer-term immune response.
Siemens Healthineers offers a robust portfolio of reliable antibody tests to support patient care
Siemens Healthineers offers both the SARS-CoV-2 Total Antibody test and the SARS-CoV-2 IgG Antibody test. The combination of these tests provides a complete picture of a patient’s serological status for the most accurate results throughout his or her continuum of care.
SARS-CoV-2 Total (COV2T) Test*
The COV2T test detects both IgM and longer-lasting IgG antibodies with 100% sensitivity† and 99.8% specificity for recent and prior infection. This test is more appropriate to detect earlier and later seroconversion.
SARS-CoV-2 IgG (COV2G) Qualitative and Semi-quantitative Test*
The COV2G test is a qualitative and semi-quantitative SARS-CoV-2 antibody test that enables clinicians to detect the level of IgG antibodies in a patient’s blood sample and assess relative changes over time. The test is more appropriate for later seroconversion. With this numerical value, clinicians will have a baseline and be better equipped to track the long-term duration of an individual’s immune response. Comparison of numerical results will help determine how the immune response develops in an individual and persists over time.
The SARS-COV-2 IgG antibody test offers 100% sensitivity† and 99.9% specificity which is critical for detecting the adaptive immune response accurately.
Neutralizing Antibodies: Why the Spike Protein?
Antibody tests from Siemens Healthineers are well-positioned to aid vaccine development efforts.
We smartly selected the receptor-binding domain (RBD) of the S1 spike antigen to detect antibodies that block the virus entry into the cells. This selection is aligned with the multiple vaccines in development that target or include the SARS-CoV-2 S1 RBD, with the goal of producing protective antibody.
The Siemens Healthineers SARS-CoV-2 antibody assays detect antibodies to the S1 RBD antigen. S1 RBD antibodies are relevant to vaccines incorporating this immunodominant region with the goal to elicit neutralizing (and therefore likely protective) antibodies in vaccinated subjects.6 The spike protein and particularly the RBD are the most common target of vaccine designs.